Fresher air for indoor pools

Alan Lewis looks at ways to improve ventilation in indoor heated public pools, based on new research that shows some toxic gases are heavier than air.

For at least a decade we have had convincing evidence that we are polluting the air in our indoor swimming pool halls (sometimes referred to as natatoriums, especially in Europe).
This malodorous pollution in poorly ventilated halls comes from trichloramine gases (NCl3 or nitrogen trichloride). Alongside this easily recognised odour are several other toxic gases which are masked by NCl3. These include chloroform (and other trihalomethanes), haloacetic acids, haloketones and cyanogen chloride. Many organic disinfection by-products (DBPs) do not cause discomfort but some have been identified as potential carcinogens, and trichloramines are considered to induce asthma.
The pollutants of most concern are trichloramines, and chloroform and other trihalomethanes. These are created in the pool in two critical chemical paths shown below.
Trichloramines – the inorganic chloramines
Free chlorine (HOCl) + body amines (urea, perspiration) => monochloramine + H2O
Free chlorine + monochloramine => dichloramine + H2O
Free chlorine + dichloramine => trichloramine (NCl3) + H2O
Blatchley and Li (2009) as well as Schmaltz et al (2011) also found that at normal pH values found in swimming pools:
Free chlorine [HOCl] + urea [CO(NH2)2] + other nitrogen compounds ==>> NCl3
Trihalomethanes (THMs) – principally chloroform (CHCl3)
It has been thought for some time that UV photolysis actually creates chloroform from (inorganic) chloramines, but Blatchley (2011) clearly showed that UV neither increases nor decreases the production of chloroform. It is now clearly understood that one of the main precursors of chloroform are skin cells.
Free chlorine (HOCl) + skin cells (on bathers or shed by bathers) ==>> CHCl3
Thus the more bathers there are the more chloroform will be created. Both of these disinfection by-products are referred to as “volatile chemicals”, which means that they have a tendency to vaporise – to change from liquid to gas.
Both exit the water as gases at the surface of the pool. This process is exacerbated by the swimmers splashing down the lane. In spas, pools and water parks with spray features they are easily aerosoled into the air above the pool surface.
Volatile trichloramine and chloroform vapours are heavy gases which accumulate in the lowest areas of the pool hall – mostly in the space below the deck level.
Pool halls with poor air quality further gravitate to the lowest point in the building – or in the case of outdoor pools the lowest point in the surroundings of the pool. Thus for example the chloramines can be smelled even in the pool car park if it is positioned below the surface of the pool. If the pool is in a building several stories high, the chloramines often find their way to the lift shaft and gravitate to lower floors with surprising ease.
Ventilation engineers have until recently followed the dictates of the relevant national standards and guidelines for the required air changes in indoor spaces. Since the unwanted gases are in the lowest areas, the conventional methods of ventilation have overlooked the need to extract the air from the lowest areas in the natatorium.
It can be assumed that the vast majority of indoor pools actually continue to this day to cause severe discomfort to teachers and staff, competitive swimmers and young children learning to swim. Even as this article was being written, Australian media announced that one of the nation’s elite swimmers was forced to retire from training in an AIS pool in Canberra due to respiratory illness.
“The prevalence of respiratory symptoms and airway hyper-responsiveness (AHR) is high in elite athletes; swimmers have one of the highest prevalences.” (Bougalt; Loubaki; Joubert et al 2012 Research Institute of Cardiology and Pneumology in Quebec Canada.)
Strategies for improving indoor air quality
Prevention
First, by removing or reducing the precursor contaminants from the circulation of the pool; and second, by:
a) Compulsory pre-immersion shower by all bathers – this removes 80 per cent to 90 per cent of urea, sweat, body amines which contribute to the immediate build-up of monochloramines when bathers first dive into the pool.
b) UV/ozone/electrolysis (Ecoline) secondary disinfection sytems.
Ultra violet (UV) photolysis is effective in reducing chloramines and some other toxic compounds but has no effect on THMs. Much of the chemistry in this application is as yet undefined and controversy still exists regarding the specifications and sizing of the units.
Ozone applications need to be carefully thought-out and detailed to avoid breakdowns. The materials selected in ozonated systems must all be of the highest quality and totally ozone resistant. Ozone sizing should not be excessive since it is dealing with relatively small residuals that need to be broken down. Ozone does oxidise skin cells captured in the filter media (and following a specific regimen not detailed here, can even affect skin cells residing in poorly circulated parts of the pool). As such it has a slight advantage over UV. Modern ozone applications using cold plasma fields for the creation of low levels of ozone which then breaks down the monochloramines.
The Ecoline electrolytic system developed by Australian Innovative Systems (AIS) not only does it do away with the need for a chlorine feed, but creates free oxidants from tap water with a low TDS (500mg/l). No added salt is needed. This process breaks down monochloramines as well – in one and the same process. As yet we have not been able to quantify this part of the system. Clearly the application of an Ecoline system to a public pool – indoor or outdoor – will reduce the chloramines by virtue of the very low levels of hypochlorous acid created. We do know that the need for UV or ozone will be markedly reduced when the Ecoline unit is working well.
c) Reducing the chlorine feed. European countries already accept that good disinfection is not dependent on high levels of free chlorine in the pool but also on very low levels of pH. Many countries already allow pH in the range 6.8 – 7.4 and free chlorine levels at 0.4 – 0.8 mg/L. Nearly always they advocate the use of secondary disinfection systems and in Australia, several states are now accepting that ORP (redox) levels of no less than 740mV are sufficient. This allows lower free chlorine residuals.
d) Designing the pool circulation so that skin cells are rapidly swept from the floor out of the pool via the wet edge gutters. The Danes have now shown that by placing the return spigots near the bottom of the walls of the pool, the water jet will sweep the skin cells on the floor towards the centre of the pool and thence up towards the surface and the wet edge gutters.
e) Using drum filters with automatic back rinsing every few hours in place of media (sand or DE) filters which retain the skin cells for many days at a time until being backwashed. Hytech filters are designed with a fine membrane which filters out all suspended solids greater than 10 micron. The membrane is automatically cleaned as soon and as often as it is clogged with suspended matter
f) Liquid chloramine /THM “stripping” in the plant room. Ole Groneborg has designed a chloramine and THM stripper in which a side stream of the pool water is sprayed into a vessel and falls through an upstream of air which carries away the noxious gases out of the building. This equipment basically emulates the “aeration” of the water in a similar fashion to the swimmer splashing down a lane. Thus 80 per cent of those gases are removed from the water that passes through the “stripper” before it returns to the pool (or balance tank).
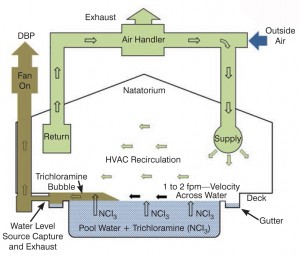
Source capture and extraction of bad air
There are three basic options:
a) Capturing the air above the water in the gutter where more gases are formed as the water splashes through the grill. This extraction should be combined with the collection of the air that is displaced in the balance tank. From there it is exhausted to the outer atmosphere.
b) At the building perimeter a duct can be run along the base of the walls where they are built close to the pool edge. In this case the duct is slightly above the lowest point (the surface of the pool) but nevertheless gets dragged over the deck between the gutter and the pool edge.
c) Under the deck. This usually entails the raising of the deck high enough to accommodate a duct beneath it so that the intake is again immediately above the surface of the pool.
Dilution of pool hall air
Conventional introduction of outside air to replace extracted air, but combined with source capture and exhaust as a retrofit which utilises or extends an existing system (see Diagram A). The refreshed air supply will force the air from one side to the other taking with it the trichloramine/THM bubble.
Destroy DBPs
The other way is to recycle heated air and introduce safe cold plasma (ozonation) destruction of the unwanted trichloramines and trihalomethanes.
With this method, the air is partially purified by passing it through a cold plasma field which converts the oxygen in the air to small amounts of ozone, which breaks down the contaminants to benign components.
In this case, the amount of fresh air introduced into the hall can be reduced because the treated air will no longer aggravate people in the hall. This method has not actually been applied yet. This concept is aimed at conserving the considerable expense of reheating the fresh air stream when the dilution is replacing all or most of the captured gases entirely.
At a time when training is in full swing for the London Olympics, we can only hope that FINA and other relevant bodies are taking steps to prevent recurrences of the respiratory illnesses that our swimmers will inevitably experience if nothing is done to improve the indoor air quality of competition venues.
This is an abridged extract from the article Catching your Breath by Alan Lewis from edition 82 of the version printed SPLASH!, published in June 2012.



